Welcome to the Hjelt Foundations
"The three Hjelt Foundations, Spina Bifida, Diabetes and Art were created because of very important events that have considerably influenced my life."
Bo Hjelt
News

The Allan and Bo Hjelt Art Foundation and Circus & Dance Finland have established a collaboration that brings international circus expertise to Finland. Through this partnership, Swedish circus director Tilde Björfors will visit Helsinki in January 2026 as part of an initiative to foster cross-border dialogue within the performing arts sector.

6-7 June 2025, ODDfest will take place in central Helsinki. The festival brings together art, business, and society through a two-day program focused on creativity and innovation. This year, the Bo and Allan Hjelt Art Foundation is partnering with ODDfest to support a keynote appearance by American professor Arthur C. Brooks.
Spina Bifida Foundation
Having lost a child due to Spina Bifida, a most traumatic experience that no family should have to go through, the Bo Hjelt Foundation for Spina Bifida in memory of Madeleine Hjelt was set up to aid research into its’ prevention.
Learn more
Diabetes Foundation
The Bo and Kerstin Hjelt Foundation for research into Diabetes II was set up because, being a diabetic himself, he wanted to contribute to research in this field as it is increasing rapidly worldwide.
Learn more
Art Foundation
The Allan and Bo Hjelt Art Foundation was created in honour of Bo Hjelt's parents. He wanted to return to Finland some pieces of art that his Finnish-born father had collected; and to create the Falsterbonaset Open Air Museum in memory of his mother’s happy times in Falsterbo (five bronze statues by the Swedish sculptor Gudmar Olovson).
Learn more
Diabetes Foundation: Our Recent Research Projects

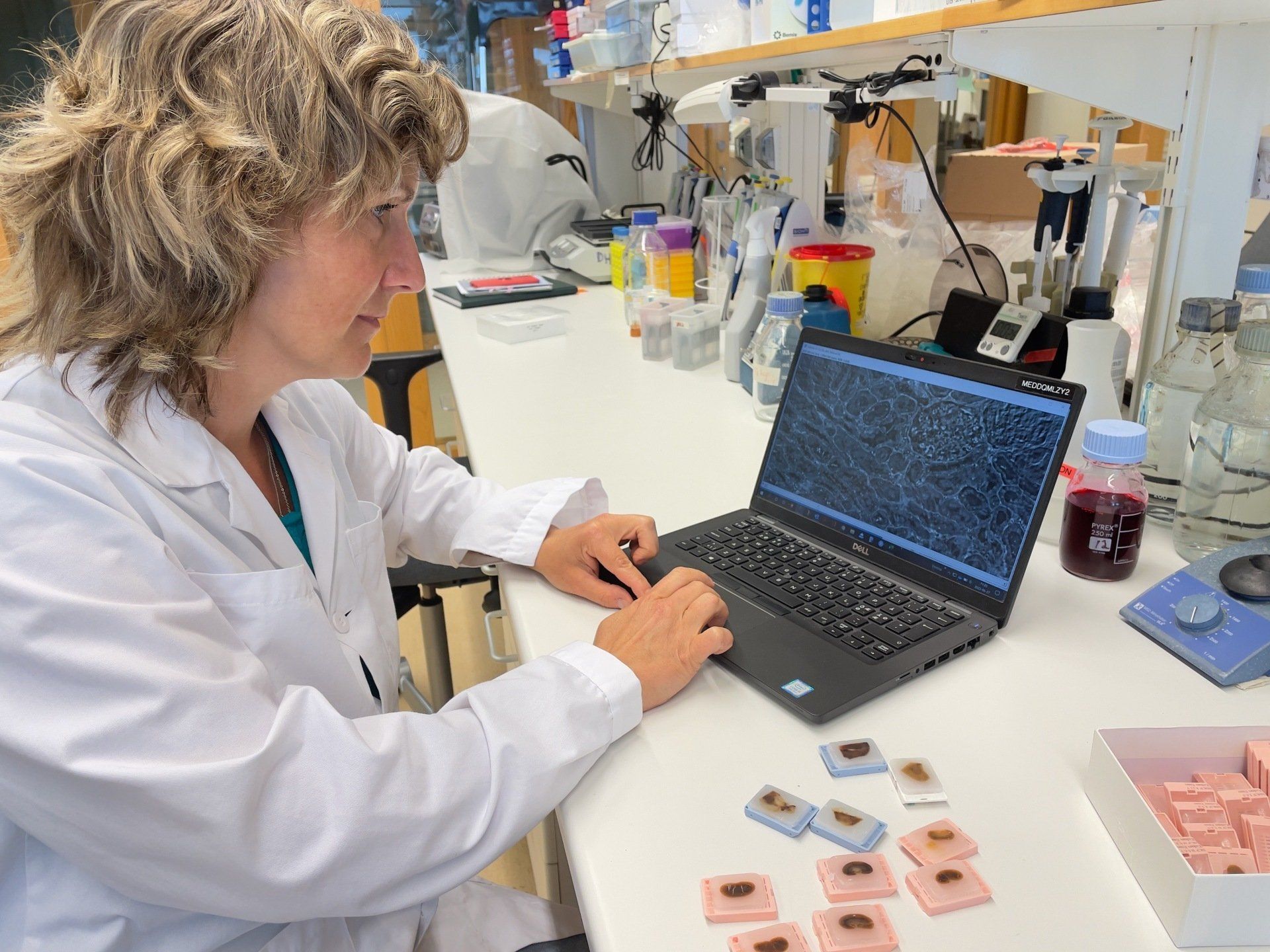
Monika Gjorgijeva Ducros, Hjelt Grant Holder 2025, University of Geneva. Background Diabetic kidney disease (DKD) is a serious complication of diabetes characterized by progressive renal dysfunction due to chronic hyperglycemia. AI showed upregulation of endoplasmic reticulum metallopeptidase 1 (ERMP1), a mediator of ER stress, in renal samples from patients with DKD. Collectively, the data suggest a protective role for ERMP1 in DKD. We aim to elucidate the interplay between renal cells with or without ERMP1 in vivo. We will use kidney-specific ERMP1 knockout mice fed either standard diet or a diabetogenic diet to induce DKD. Around 403.000 patients per year are diagnosed with renal carcinomas worldwide. Renal carcinomas are promoted by several factors such as obesity/diabetes, chronic kidney disease, smoking, toxin exposure, genetic predispositions and high blood pressure. This diverse array of risk factors leads to very strong differences in terms of tumor morphology, mutational profile and metabolic status and testifies for the need to deepen our understanding of the pathological processes involved in this disease in order to develop novel therapeutic approaches to fight renal cancer. Dr Gjorgjieva’s research aims therefore at deciphering the different molecular mechanisms involved in the incidence and progression of renal cancer . She is working on the development of an in vivo model of renal carcinoma that better mimics the pathology observed in humans and might open new therapeutic avenues.

Therapeutic potential of S100A10 Inhibition in Hepatic Insulin Resistance, MASLD and Type 2 Diabetes
Etienne Delangre, Hjelt Grant Holder 2024, University of Geneva. Therapeutic potential of S100A10 Inhibition in Hepatic Insulin Resistance, MASLD and Type 2 Diabetes